Research
Below are research products, including journal articles, oral presentations, and poster presentations, that I have been involved with, arranged in reverse chronological order. All items listed here were either peer reviewed or invited. Use the search box below to filter articles by type (article, oral, poster), co-author, title, or abstract.
2025
- poster
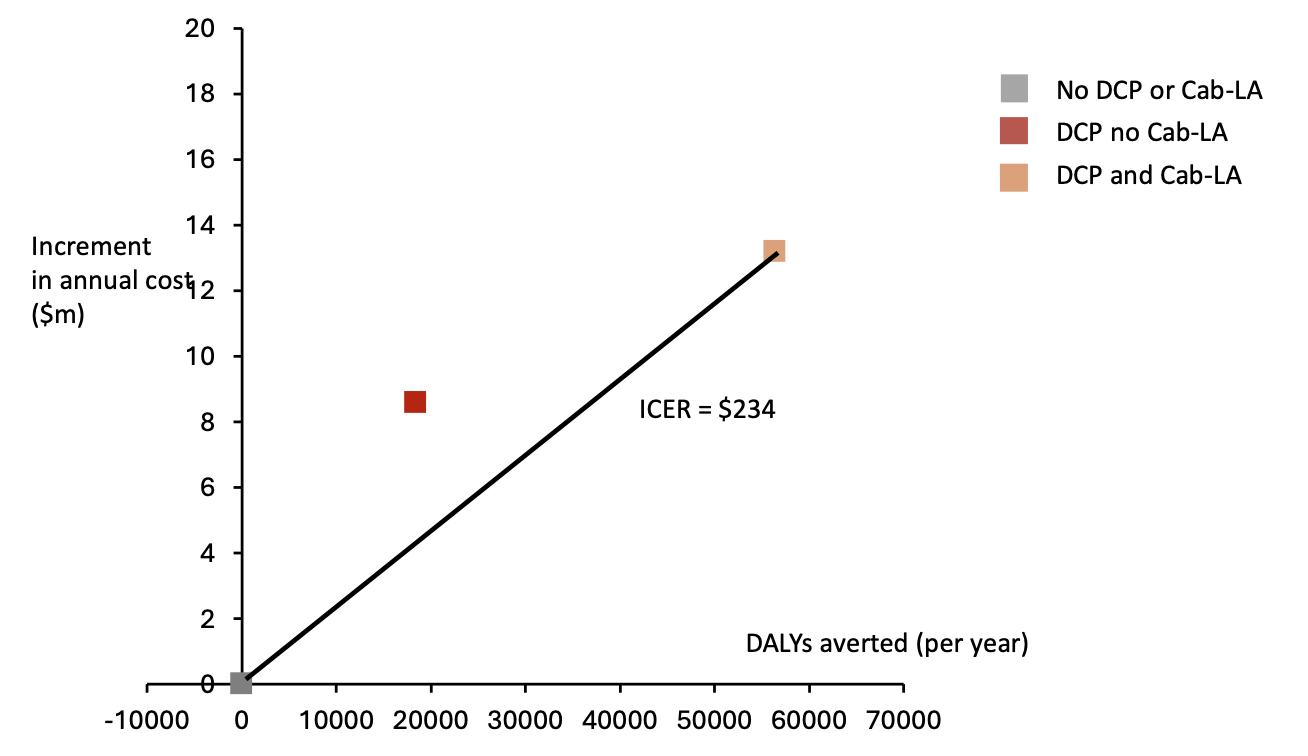 Dynamic Choice HIV Prevention with Cabotegravir (Cab-LA): a model-based cost-effectiveness analysisAndrew Phillips, Matthew Hickey*, Starley Shade, Jane Kabami, James Ayieko, Elijah Kakande, Laura Balzer, Nicole Sutter, Loveleen Bansi-Matharu, Jennifer Smith, John Schrom, Gabriel Chamie, Diane Havlir, Moses Kamya, and Maya Petersen2025
Dynamic Choice HIV Prevention with Cabotegravir (Cab-LA): a model-based cost-effectiveness analysisAndrew Phillips, Matthew Hickey*, Starley Shade, Jane Kabami, James Ayieko, Elijah Kakande, Laura Balzer, Nicole Sutter, Loveleen Bansi-Matharu, Jennifer Smith, John Schrom, Gabriel Chamie, Diane Havlir, Moses Kamya, and Maya Petersen2025Background: The SEARCH dynamic choice HIV prevention (DCP) intervention, including structured choice of biomedical prevention modality (oral PrEP, PEP, Cab-LA) with option to switch over time, increased time covered by biomedical prevention and reduced HIV incidence among men and women in randomized trials in Kenya and Uganda. We use HIV Synthesis, an existing individual-based HIV simulation model, to project long-term impact and cost-effectiveness of this approach in Africa. Methods: By sampling parameter values at the start of each model run we created 500 “setting-scenarios” reflecting uncertainty in assumptions and a range of characteristics representing those observed across east and southern Africa. For each setting-scenario, 3 policies over 10 and 50 years beginning 2024 were considered: (i) status quo, (ii) DCP with oral PrEP, PEP and condoms; (iii) DCP also including Cab-LA. We used empirical data from the DCP trials to inform the intervention’s impact on PrEP/PEP initiation and persistence, and hence HIV incidence, as well as costs. Cost-effectiveness analysis was from a healthcare perspective (using cost-effectiveness threshold US500 per DALY averted). We calculated net DALYs averted for DCP with and without Cab-LA, relative to status quo, to reflect predicted policy impact on overall population burden of disease, and incremental cost effectiveness ratio (ICER). Results: The DCP interventions are predicted to increase the proportion of people with a current PrEP/PEP indication who take PrEP/PEP and hence reduce HIV incidence relative to status quo over 10 years; median (90% range) across setting-scenarios HIV incidence rate ratios of 0.90 (0.64 – 1.19) and 0.66 (0.42 – 0.96) for DCP without and with Cab-LA, respectively. In the context of 10 million adults, both DCP policies lead to DALYs being averted (mean -12,500 and -35,000 for DCP without and with Cab-LA, respectively) but also to greater costs (+8.4m and +13.6m for DCP without and with Cab-LA, respectively). Over 50 years, DCP without Cab-LA is not predicted to be cost-effective (+4200 mean net DALYs; ICER if Cab-LA inclusion is not an option = 672/DALY averted. DCP with Cab-LA incurs the lowest mean net DALYs (-7700 per year over 50 years; ICER compared with status quo = $389), and is the cost-effective policy. Conclusions: Offering structured PrEP/PEP choice, including Cab-LA, could reduce HIV incidence by one-third over 10 years and is likely cost-effective across settings in east and southern Africa.
- poster
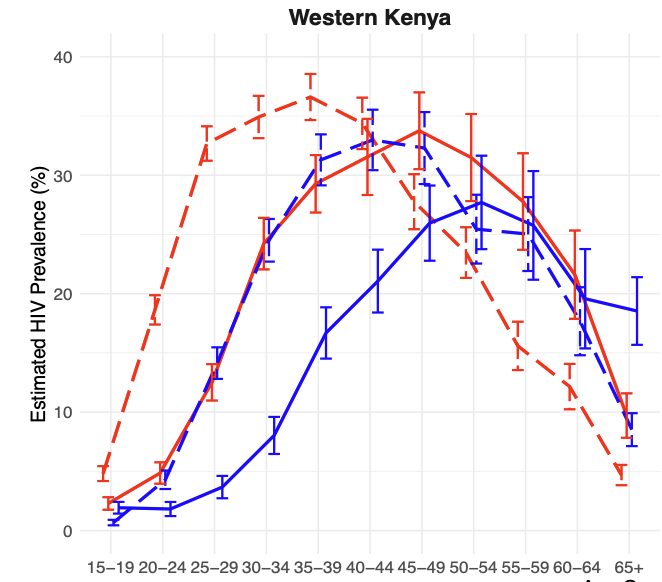 Changes in the HIV Care Cascade and Prevalence from 2013 to 2023 in Rural Kenya and Uganda in SEARCHGabriel Chamie*, Colette Inviolata, Helen Sunday, George Agengo, Wafula E Mugoma, Matthew Hickey, Douglas Black, John Schrom, Elijah Kakande, Jane Kabami, James Ayieko, Maya Petersen, Diane Havlir, Moses Kamya, and Laura Balzer2025
Changes in the HIV Care Cascade and Prevalence from 2013 to 2023 in Rural Kenya and Uganda in SEARCHGabriel Chamie*, Colette Inviolata, Helen Sunday, George Agengo, Wafula E Mugoma, Matthew Hickey, Douglas Black, John Schrom, Elijah Kakande, Jane Kabami, James Ayieko, Maya Petersen, Diane Havlir, Moses Kamya, and Laura Balzer2025Background: We sought to understand how the HIV epidemic is evolving over time by evaluating HIV prevalence and changes in status awareness and antiretroviral therapy (ART) use over 10 years in two high-prevalence, rural regions in East Africa. Methods: In 2013 and 2023 in rural communities ( 5,000 adults/community) in western (W) Kenya and southwestern (SW) Uganda, we conducted universal HIV screening of adult (≥15 years) residents at enrollment in two SEARCH population-level cluster randomized trials (NCT01864603; NCT05768763). Using TMLE and accounting for clustering by community, we estimated age- and sex-specific HIV prevalence, status awareness among persons with HIV, and ART use if living with HIV and aware of status. Results: In 2013, in 22 communities (12 W-Kenya, 10 SW-Uganda), we ascertained HIV status among 90,467/101,200 (89%) residents. In 2023, among 8 different communities from the same regions (4 W-Kenya, 4 SW-Uganda), we ascertained HIV status among 38,360/42,866 (89%) residents. In W-Kenya, HIV prevalence decreased from 18.7% in 2013 to 12.7% in 2023 (p<0.001). From 2013 to 2023, there were large, significant reductions in HIV prevalence among women aged 15-44 and men aged 20-49 years (Figure), but significant increases among women ≥45. Overall, from 2013 to 2023, HIV status awareness increased from 64% to 90% (p<0.001), and ART use from 81% to 94% (p<0.001), with large, significant increases across all age-sex strata, and greatest gains among men and youth. In contrast, in SW-Uganda, HIV prevalence increased from 6.4% in 2013 to 7.3% in 2023 (p=0.007). There were small, significant reductions in prevalence among women aged 15-29, and men 20-39 (Figure), but prevalence increased among women and men ≥40 years. Overall, from 2013 to 2013, HIV status awareness increased from 57% to 68% (p<0.001), and ART use from 82% to 87% (p=0.002); however, these improvements were not uniform by age and sex in SW-Uganda. Conclusions: Population-level prevalence data are consistent with a substantial decline in HIV incidence in W-Kenya, particularly among young adults, but not SW-Uganda. Persistently low status awareness, well below the 95% target, over the past 10 years in SW-Uganda may explain this disparity and suggests that efforts to improve status awareness are needed to achieve further reductions in HIV incidence.
2024
- article
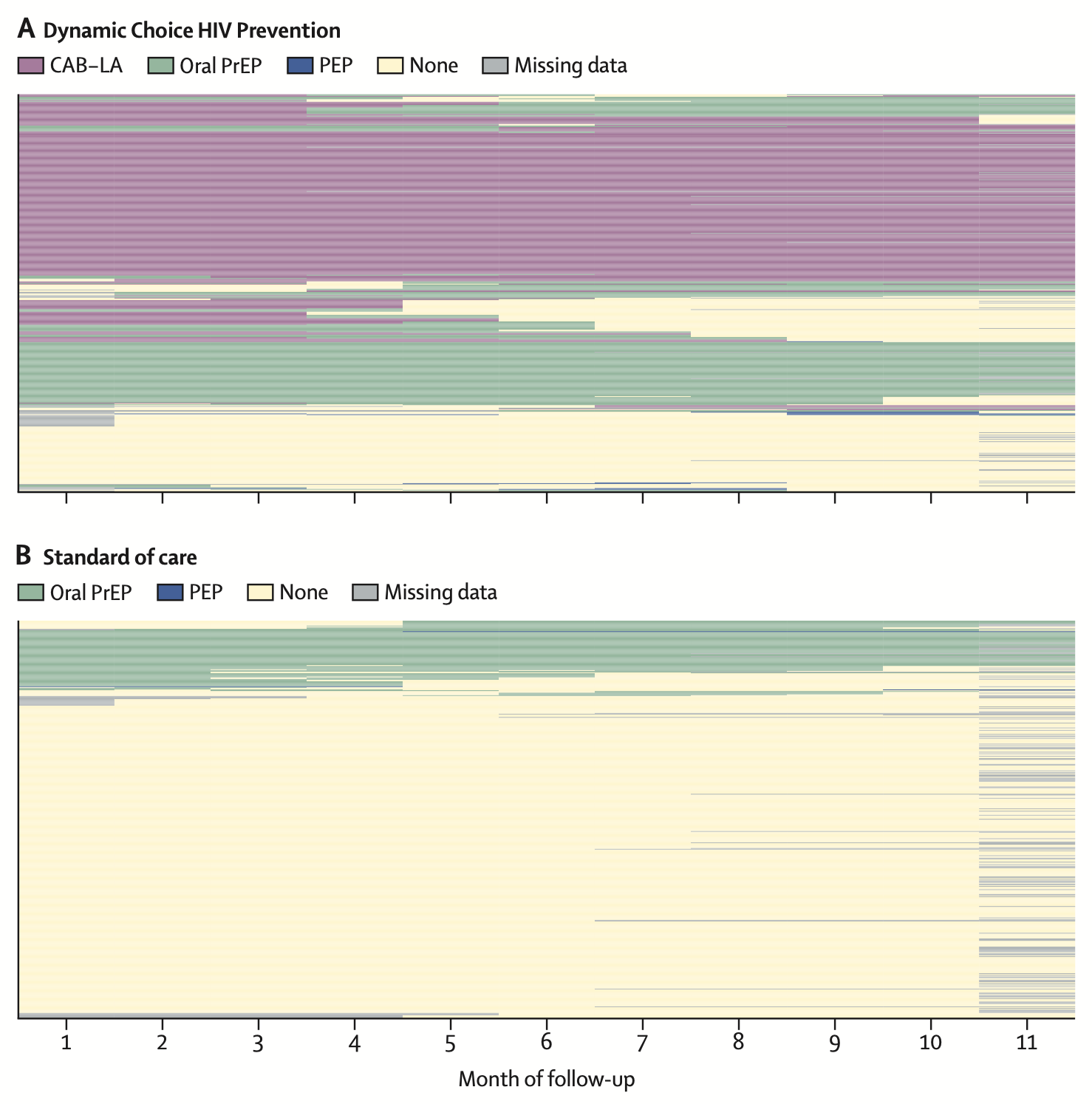 Dynamic choice HIV prevention with cabotegravir long-acting injectable in rural Uganda and Kenya: a randomised trial extensionMoses R Kamya, Laura B Balzer, James Ayieko, Jane Kabami, Elijah Kakande, Gabriel Chamie, Nicole Sutter, Helen Sunday, Janice Litunya, Joshua Schwab, John Schrom, Melanie Bacon, Catherine A Koss, Alex R Rinehart, Maya Petersen, and Diane V HavlirThe Lancet HIV, 2024
Dynamic choice HIV prevention with cabotegravir long-acting injectable in rural Uganda and Kenya: a randomised trial extensionMoses R Kamya, Laura B Balzer, James Ayieko, Jane Kabami, Elijah Kakande, Gabriel Chamie, Nicole Sutter, Helen Sunday, Janice Litunya, Joshua Schwab, John Schrom, Melanie Bacon, Catherine A Koss, Alex R Rinehart, Maya Petersen, and Diane V HavlirThe Lancet HIV, 2024Background: HIV infections are ongoing globally despite efficacious biomedical prevention options. We sought to determine whether an HIV prevention package providing choice of daily pills or long-acting injectable cabotegravir and opportunities to change prevention options could increase biomedical prevention coverage and reduce new HIV infections. Methods: This study was an extension of three randomised trials that used SEARCH dynamic choice HIV prevention to recruit adults (aged ≥15 years) at risk for HIV from antenatal, outpatient, and community settings in rural Uganda and Kenya. In this 48-week open-label extension, participants maintained their original (1:1) randomisation group; the option to choose cabotegravir long-acting injectable was added for intervention participants. Inclusion criteria for the extension were previous enrolment in a SEARCH dynamic choice HIV prevention trial, negative HIV rapid test, and residence in study region. The intervention provided person-centred choice of oral pre-exposure prophylaxis (PrEP) or post-exposure HIV prophylaxis (PEP) or cabotegravir long-acting injectable, with the option to switch according to participant preference. The control provided standard-of-care access to oral PrEP and PEP, but not cabotegravir long-acting injectable. Biomedical prevention coverage (proportion of follow-up covered by oral PrEP, PEP, or cabotegravir long-acting injectable; primary outcome) and HIV incidence (secondary outcome) were compared between groups using targeted minimum loss-based estimation. The trial (NCT05549726) is closed to recruitment. Findings: Of 1534 participants initially randomly assigned (from April 15, 2021 to Sept 29, 2022), 984 (487 in the intervention group and 497 in the standard-of-care group) reconsented to the extension (from Jan 2 to March 3, 2023). The mean proportion of follow-up covered by biomedical HIV prevention was 69·7% (95% CI 64·9–74·5) in the intervention group versus 13·3% (10·2–16·3) in the standard-of-care group, corresponding to an absolute difference of 56·4 percentage points (95% CI 50·8–62·1; p<0·0001). The intervention significantly improved coverage across prespecified subgroups (sex and age groups). During the study, 274 (56%) of 485 intervention participants used cabotegravir long-acting injectable, 255 (53%) used oral PrEP, and ten (2%) used PEP. Among cabotegravir long-acting injectable initiators, 118 (43%) of 274 were not previously using oral PrEP or PEP. There were seven incident HIV infections in 390 person-years of follow-up in the standard-of-care group and no infections in 400 person-years of follow-up in the intervention group (incidence rate difference per 100 person-years 1·8, 95% CI 0·4–3·2; p=0·014). Interpretation: Offering people the choice of HIV biomedical prevention options including cabotegravir long-acting injectable in a flexible model can increase prevention coverage and reduce incident HIV infections. HIV programmes should support dynamic choice HIV prevention programmes that include effective oral and injectable long-acting products.
- oral
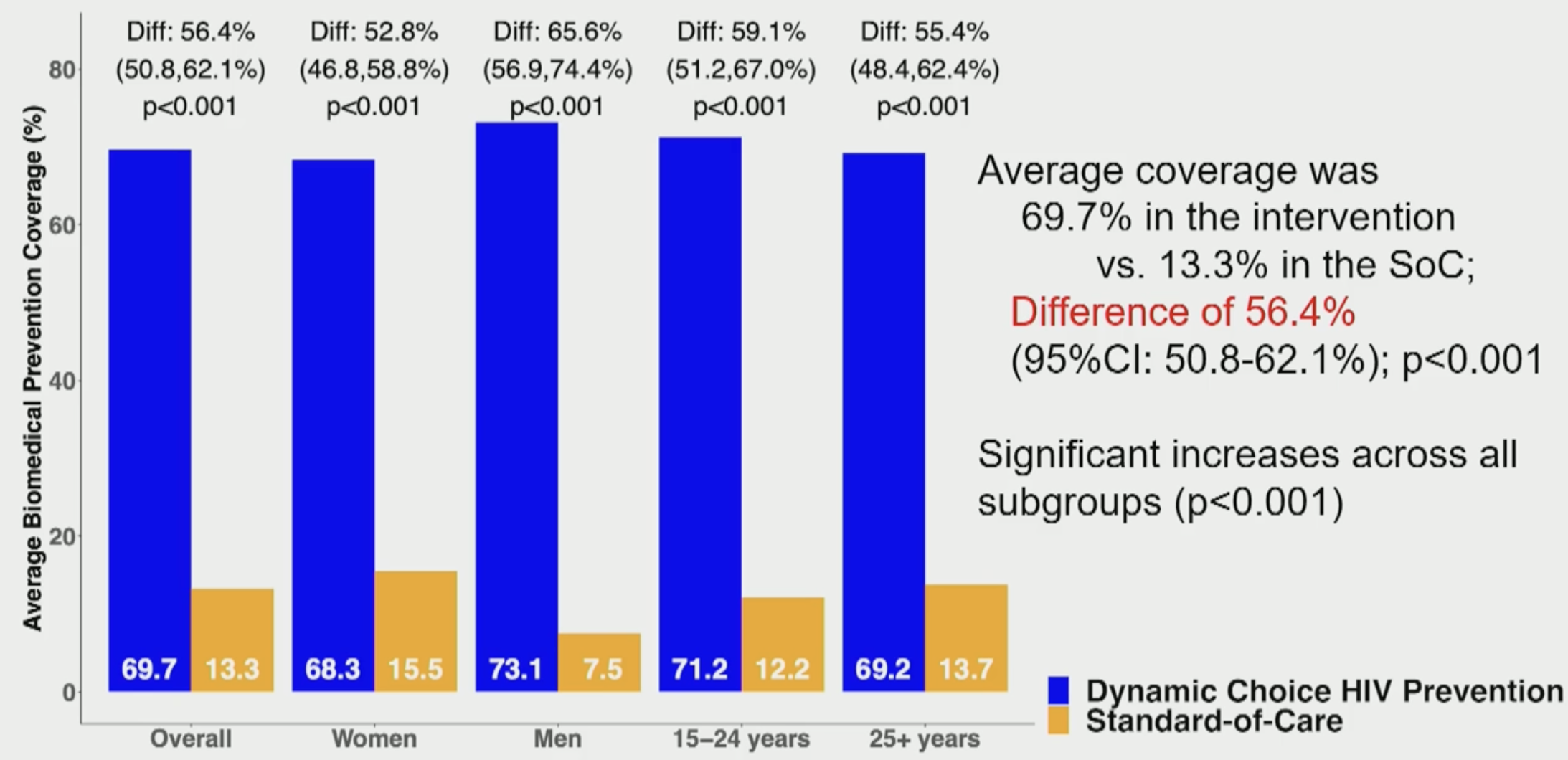 Randomized Trial of SEARCH Dynamic Choice HIV Prevention Including Injectable Cabotegravir (CAB-LA)Moses Kamya*, Laura Balzer, James Ayieko, Jane Kabami, Elijah Kakande, Gabriel Chamie, Nicole Sutter, Helen Sunday, John Schrom, Melanie Bacon, Catherine Koss, Alex Rinehart, Maya Petersen, and Diane HavlirIn CROI, 2024
Randomized Trial of SEARCH Dynamic Choice HIV Prevention Including Injectable Cabotegravir (CAB-LA)Moses Kamya*, Laura Balzer, James Ayieko, Jane Kabami, Elijah Kakande, Gabriel Chamie, Nicole Sutter, Helen Sunday, John Schrom, Melanie Bacon, Catherine Koss, Alex Rinehart, Maya Petersen, and Diane HavlirIn CROI, 2024Background: We hypothesized that an oral PrEP, PEP and CAB-LA biomedical prevention package with structured choice between products and opportunities to switch would increase biomedical prevention coverage compared to standard-of-care (SoC) among men and women at risk for HIV in rural Uganda and Kenya. Methods: Participants were recruited from three randomized studies of the SEARCH dynamic choice HIV prevention (DCP) intervention vs SoC in antenatal clinics, outpatient departments and the community. Eligible participants were > 15 years and reported risk of acquiring HIV. Participants in the SoC arm had access to oral PrEP (TDF/XTC) and PEP (TLD) at local Ministry of Health (MoH) clinics. The SEARCH DCP model included person-centered, structured choice between oral PrEP, PEP (MoH-supplied) or CAB-LA (study-supplied at MoH clinics) and the ability to switch between or stop products over time based on patient product preference and risk. Primary outcome was biomedical covered time over 48 weeks (proportion of follow-up covered by PrEP/PEP/CAB-LA), assessed via study logs and self-report; secondary outcomes included coverage during periods of retrospectively self-assessed HIV risk and incident HIV infections. Results: We enrolled 984 participants (487 DCP; 497 SoC). 73% were women, 30% aged 15-24. Mean biomedical covered time was higher in DCP (69.7%) vs. SoC (13.3%), a difference of 56.4% (95% CI 50.8-62.1%; p<0.001). Biomedical covered time with DCP vs SoC was 65.6% and 52.8% higher for men and women, respectively. Intervention effect on coverage during periods at risk of HIV was larger; mean at-risk covered time was 76.5% in the DCP arm vs. 16.2% in SoC (difference 60.2%; 95%CI: 53.8-66.6%; p<0.001). In the DCP arm, 56%, 53%, 2% ever used CAB-LA, PrEP or PEP, respectively. 43% of persons who used CAB-LA were not using prior oral PrEP or PEP, showing benefit of adding the CAB- LA option. 28% and 0.4% of participants used at least 2 different products in the DCP and SoC arms, respectively. There were 7 participants who acquired HIV infection and one perinatal transmission in the SoC arm (incidence rate: 1.8%) and 0 in the DCP arm (p=0.01). Conclusions: In the first randomized study of a person-centered model offering structured choice between CAB-LA, oral PREP and PEP with option to change over time, enrolling both women and men at risk of HIV, the SEARCH DCP intervention increased biomedical covered time by >5 fold to 69.7% and reduced HIV incidence to 0% compared to 1.8% in standard-of-care.
2023
- article
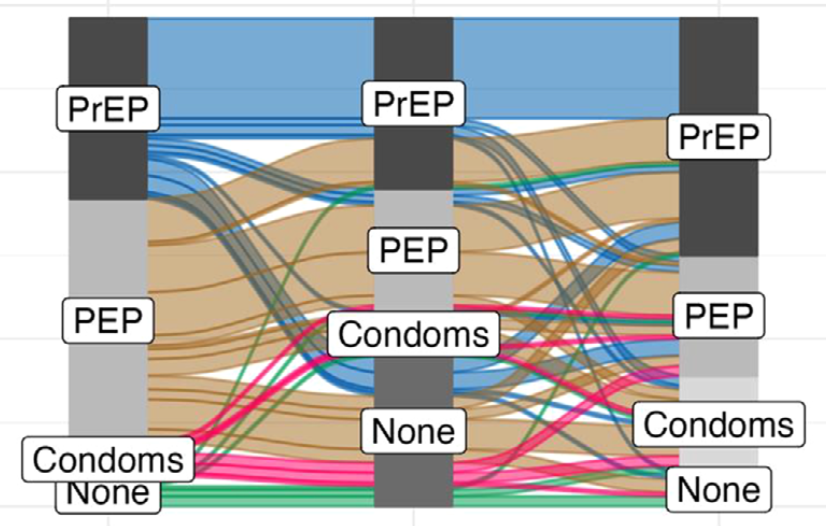 A community-based dynamic choice model for HIV prevention improves PrEP and PEP coverage in rural Uganda and Kenya: a cluster randomized trialElijah R Kakande, James Ayieko, Helen Sunday, Edith Biira, Marilyn Nyabuti, George Agengo, Jane Kabami, Colette Aoko, Hellen N Atuhaire, Norton Sang, Asiphas Owaranganise, Janice Litunya, Erick W Mugoma, Gabriel Chamie, James Peng, John Schrom, Melanie C Bacon, Moses R Kamya, Diane V Havlir, Maya L Petersen, and Laura B BalzerJournal of the International AIDS Society, 2023
A community-based dynamic choice model for HIV prevention improves PrEP and PEP coverage in rural Uganda and Kenya: a cluster randomized trialElijah R Kakande, James Ayieko, Helen Sunday, Edith Biira, Marilyn Nyabuti, George Agengo, Jane Kabami, Colette Aoko, Hellen N Atuhaire, Norton Sang, Asiphas Owaranganise, Janice Litunya, Erick W Mugoma, Gabriel Chamie, James Peng, John Schrom, Melanie C Bacon, Moses R Kamya, Diane V Havlir, Maya L Petersen, and Laura B BalzerJournal of the International AIDS Society, 2023Introduction: Optimizing HIV prevention may require structured approaches for providing client-centred choices as well as community-based entry points and delivery. We evaluated the effect of a dynamic choice model for HIV prevention, delivered by community health workers (CHWs) with clinician support, on the use of biomedical prevention among persons at risk of HIV in rural East Africa. Methods: We conducted a cluster randomized trial among persons (≥15 years) with current or anticipated HIV risk in 16 villages in Uganda and Kenya (SEARCH; NCT04810650). The intervention was a client-centred HIV prevention model, including (1) structured client choice of product (pre-exposure prophylaxis [PrEP] or post-exposure prophylaxis [PEP]), service location (clinic or out-of-clinic) and HIV testing modality (self-test or rapid test), with the ability to switch over time; (2) a structured assessment of patient barriers and development of a personalized support plan; and (3) phone access to a clinician 24/7. The intervention was delivered by CHWs and supported by clinicians who oversaw PrEP and PEP initiation and monitoring. Participants in control villages were referred to local health facilities for HIV prevention services, delivered by Ministry of Health staff. The primary outcome was biomedical prevention coverage: a proportion of 48-week follow-up with self-reported PrEP or PEP use. Results: From May to July 2021, we enrolled 429 people (212 intervention; 217 control): 57% women and 35% aged 15–24 years. Among intervention participants, 58% chose PrEP and 58% chose PEP at least once over follow-up; self-testing increased from 52% (baseline) to 71% (week 48); ≥98% chose out-of-facility service delivery. Among 413 (96%) participants with the primary outcome ascertained, average biomedical prevention coverage was 28.0% in the intervention versus 0.5% in the control: a difference of 27.5% (95% CI: 23.0–31.9%, p<0.001). Impact was larger during periods of self-reported HIV risk: 36.6% coverage in intervention versus 0.9% in control, a difference of 35.7% (95% CI: 27.5–43.9, p<0.001). Intervention effects were seen across subgroups defined by sex, age group and alcohol use. Conclusions: A client-centred dynamic choice HIV prevention intervention, including the option to switch between products and CHW-based delivery in the community, increased biomedical prevention coverage by 27.5%. However, substantial person-time at risk of HIV remained uncovered.
- article
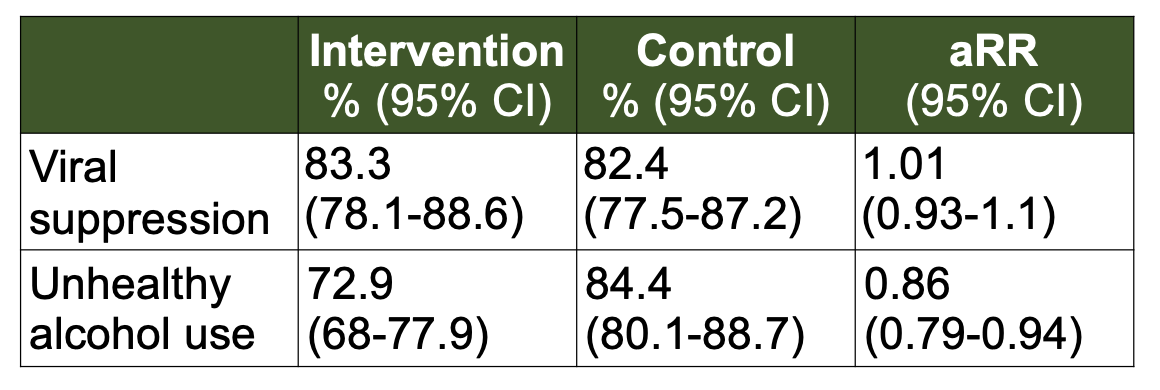
 Effect of a brief alcohol counselling intervention on HIV viral suppression and alcohol use among persons with HIV and unhealthy alcohol use in Uganda and Kenya: a randomized controlled trialSarah B Puryear, Florence Mwangwa, Fred Opel, Gabriel Chamie, Laura B Balzer, Jane Kabami, James Ayieko, Asiphas Owaraganise, Elijah Kakande, George Agengo, Elizabeth Bukusi, Stella Kabageni, Daniel Omoding, Melanie Bacon, John Schrom, Sarah Woolf-King, Maya L. Petersen, Diane V. Havlir, Moses Kamya, and Judy H. HahnJournal of the International AIDS Society, 2023
Effect of a brief alcohol counselling intervention on HIV viral suppression and alcohol use among persons with HIV and unhealthy alcohol use in Uganda and Kenya: a randomized controlled trialSarah B Puryear, Florence Mwangwa, Fred Opel, Gabriel Chamie, Laura B Balzer, Jane Kabami, James Ayieko, Asiphas Owaraganise, Elijah Kakande, George Agengo, Elizabeth Bukusi, Stella Kabageni, Daniel Omoding, Melanie Bacon, John Schrom, Sarah Woolf-King, Maya L. Petersen, Diane V. Havlir, Moses Kamya, and Judy H. HahnJournal of the International AIDS Society, 2023Introduction: Unhealthy alcohol use significantly contributes to viral non-suppression among persons with HIV (PWH). It is unknown whether brief behavioural interventions to reduce alcohol use can improve viral suppression among PWH with unhealthy alcohol use in sub-Saharan Africa (SSA). Methods: As part of the SEARCH study (NCT04810650), we conducted an individually randomized trial in Kenya and Uganda of a brief, skills-based alcohol intervention among PWH with self-reported unhealthy alcohol use (Alcohol Use Disorders Identification Test–Consumption [AUDIT-C], prior 3 months, ≥3/female; ≥4/male) and at risk of viral non-suppression, defined as either recent HIV viral non-suppression (≥400 copies/ml), missed visits, out of care or new diagnosis. The intervention included baseline and 3-month in-person counselling sessions with interim booster phone calls every 3 weeks. The primary outcome was HIV viral suppression (<400 copies/ml) at 24 weeks, and the secondary outcome was unhealthy alcohol use, defined by AUDIT-C or phosphatidylethanol (PEth), an alcohol biomarker, ≥50 ng/ml at 24 weeks. Results: Between April and September 2021, 401 persons (198 intervention, 203 control) were enrolled from HIV clinics in Uganda (58%) and Kenya (27%) and alcohol-serving venues in Kenya (15%). At baseline, 60% were virally suppressed. Viral suppression did not differ between arms at 24 weeks: suppression was 83% in intervention and 82% in control arms (RR: 1.01, 95% CI: 0.93–1.1). Among PWH with baseline viral non-suppression, 24-week suppression was 73% in intervention and 64% in control arms (RR 1.15, 95% CI: 0.93–1.43). Unhealthy alcohol use declined from 98% at baseline to 73% in intervention and 84% in control arms at 24 weeks (RR: 0.86, 95% CI: 0.79–0.94). Effects on unhealthy alcohol use were stronger among women (RR 0.70, 95% CI: 0.56–0.88) than men (RR 0.93, 95% CI: 0.85–1.01) and among participants with a baseline PEth⩽200 ng/ml (RR 0.68, 95% CI: 0.53–0.87) versus >200 ng/ml (RR 0.97, 95% CI: 0.92–1.02). Conclusions: In a randomized trial of 401 PWH with unhealthy alcohol use and risk for viral non-suppression, a brief alcohol intervention reduced unhealthy alcohol use but did not affect viral suppression at 24 weeks. Brief alcohol interventions have the potential to improve the health of PWH in SSA by reducing alcohol use, a significant driver of HIV-associated co-morbidities.
- article
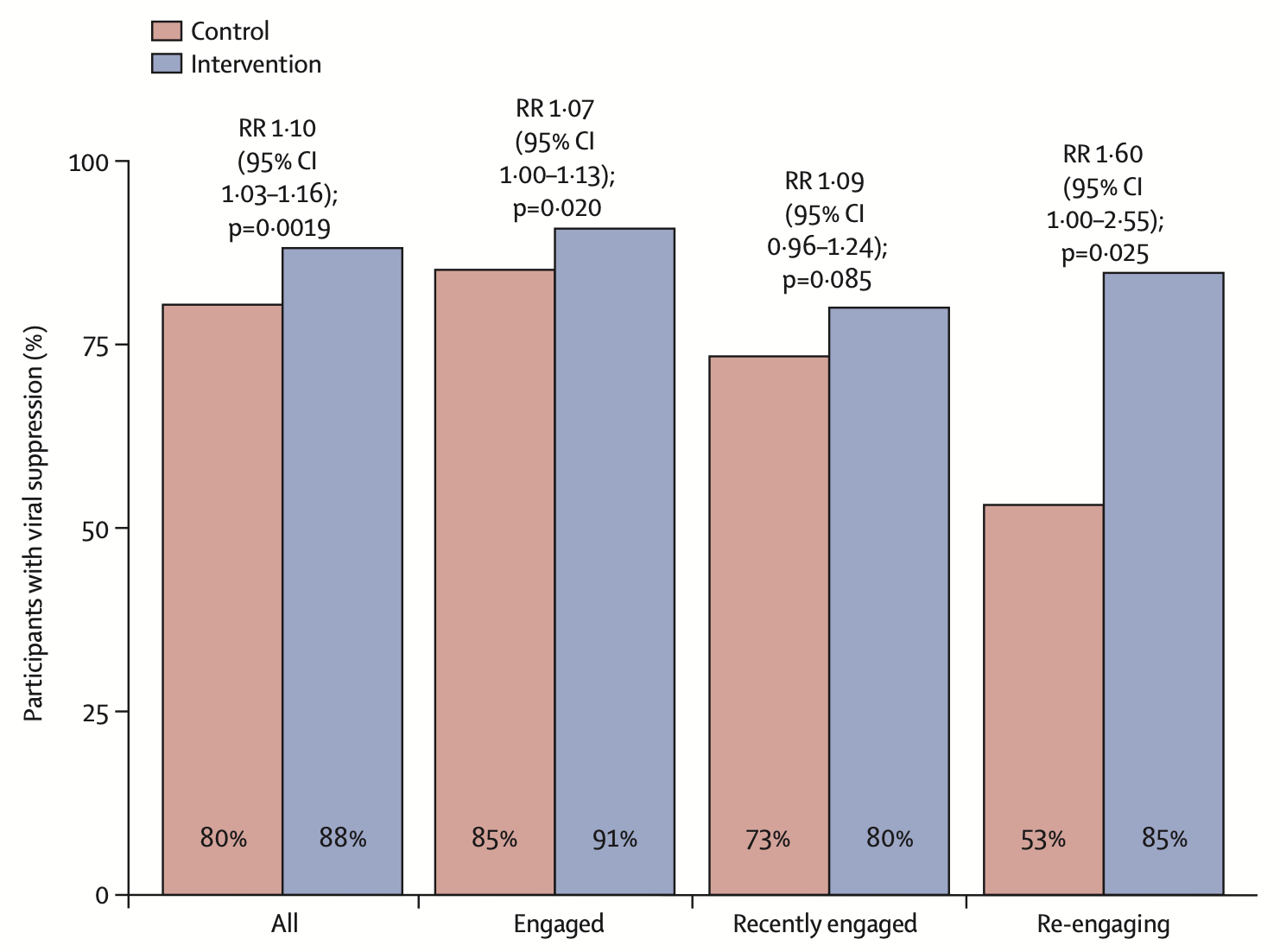 A multilevel health system intervention for virological suppression in adolescents and young adults living with HIV in rural Kenya and Uganda (SEARCH-Youth): a cluster randomised trialTheodore Ruel, Florence Mwangwa, Laura B Balzer, James Ayieko, Marilyn Nyabuti, Wafula Erick Mugoma, Jane Kabami, Brian Kamugisha, Douglas Black, Bridget Nzarubara, Fred Opel, John Schrom, George Agengo, Janet Nakigudde, Hellen N Atuhaire, Josh Schwab, James Peng, Carol Camlin, Starley B Shade, Elizabeth Bukusi, Bill G Kapogiannis, Edwin Charlebois, Moses R Kamya, and Diane HavlirThe Lancet HIV, 2023
A multilevel health system intervention for virological suppression in adolescents and young adults living with HIV in rural Kenya and Uganda (SEARCH-Youth): a cluster randomised trialTheodore Ruel, Florence Mwangwa, Laura B Balzer, James Ayieko, Marilyn Nyabuti, Wafula Erick Mugoma, Jane Kabami, Brian Kamugisha, Douglas Black, Bridget Nzarubara, Fred Opel, John Schrom, George Agengo, Janet Nakigudde, Hellen N Atuhaire, Josh Schwab, James Peng, Carol Camlin, Starley B Shade, Elizabeth Bukusi, Bill G Kapogiannis, Edwin Charlebois, Moses R Kamya, and Diane HavlirThe Lancet HIV, 2023Background: Social and cognitive developmental events can disrupt care and medication adherence among adolescents and young adults living with HIV in sub-Saharan Africa. We hypothesised that a dynamic multilevel health system intervention helping adolescents and young adults and their providers navigate life-stage related events would increase virological suppression compared with standard care. Methods: We did a cluster randomised, open-label trial of young individuals aged 15–24 years with HIV and receiving care in eligible clinics (operated by the government and with ≥25 young people receiving care) in rural Kenya and Uganda. After clinic randomisation stratified by region, patient population, and previous participation in the SEARCH trial, participants in intervention clinics received life-stage-based assessment at routine visits, flexible clinic access, and rapid viral load feedback. Providers had a secure mobile platform for interprovider consultation. The control clinics followed standard practice. The primary, prespecified endpoint was virological suppression (HIV RNA <400 copies per mL) at 2 years of follow-up among participants who enrolled before Dec 1, 2019, and received care at the study clinics. This trial is registered with ClinicalTrials.gov, NCT03848728, and is closed to recruitment. Findings: 28 clinics were enrolled and randomly assigned (14 control, 14 intervention) in January, 2019. Between March 14, 2019, and Nov 26, 2020, we recruited 1988 participants at the clinics, of whom 1549 were included in the analysis (785 at intervention clinics and 764 at control clinics). The median participant age was 21 years (IQR 19–23) and 1248 (80·6%) of 1549 participants were female. The mean proportion of participants with virological suppression at 2 years was 88% (95% CI 85–92) for participants in intervention clinics and 80% (77–84) for participants in control clinics, equivalent to a 10% beneficial effect of the intervention (risk ratio [RR] 1·10, 95% CI 1·03–1·16; p=0·0019). The intervention resulted in increased virological suppression within all subgroups of sex, age, and care status at baseline, with greatest improvement among those re-engaging in care (RR 1·60, 95% CI 1·00–2·55; p=0·025). Interpretation: Routine and systematic life-stage-based assessment, prompt adherence support with rapid viral load testing, and patient-centred, flexible clinic access could help bring adolescents and young adults living with HIV closer towards a goal of universal virological suppression.
- poster
 Randomized Trial of Brief Alcohol Intervention for Viral Suppression and Alcohol UseSarah Puryear*, Florence Mwangwa, Fred Opel, Gabriel Chamie, Laura Balzer, Jane Kabami, James Ayieko, Elijah Kakande, John Schrom, Melanie Bacon, Sarah Woolf-King, Maya Petersen, Diane Havlir, Moses Kamya, and Judith HahnIn CROI, 2023
Randomized Trial of Brief Alcohol Intervention for Viral Suppression and Alcohol UseSarah Puryear*, Florence Mwangwa, Fred Opel, Gabriel Chamie, Laura Balzer, Jane Kabami, James Ayieko, Elijah Kakande, John Schrom, Melanie Bacon, Sarah Woolf-King, Maya Petersen, Diane Havlir, Moses Kamya, and Judith HahnIn CROI, 2023A RCT of a brief alcohol counseling intervention vs. control showed no effects on viral suppression, but did reduce unhealthy alcohol use among PWH with unhealthy alcohol use and high risk of HIV viremia in East Africa
- article
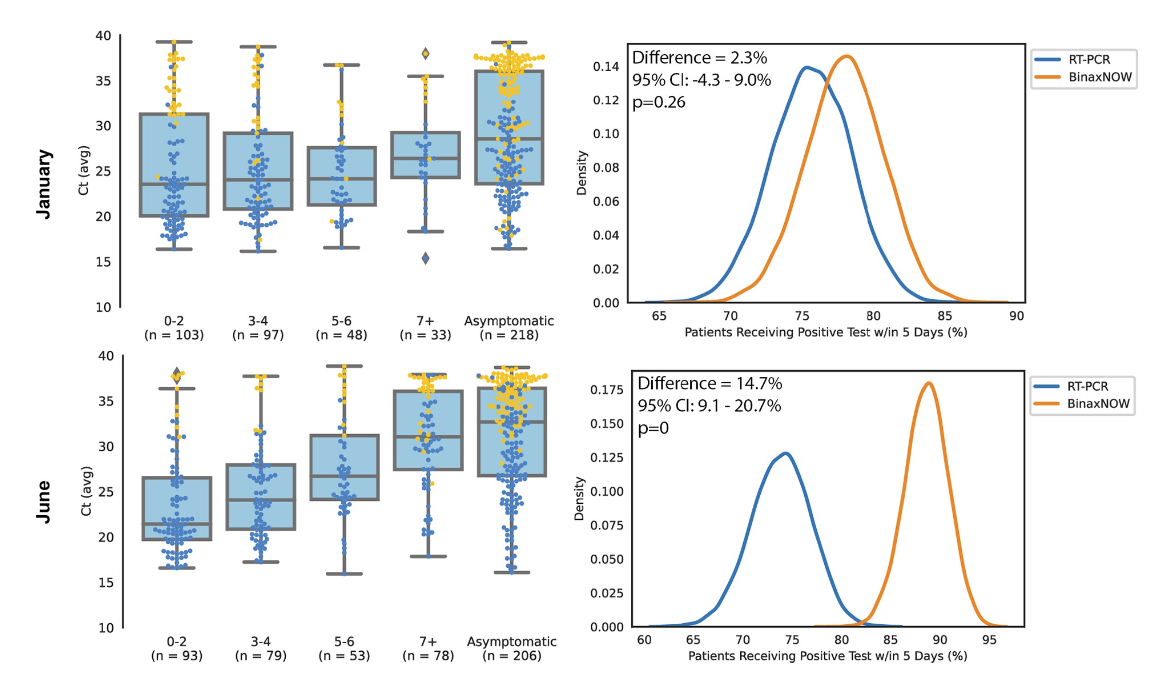 Field assessment of BinaxNOW antigen tests as COVID-19 treatment entry point at a community testing site in San Francisco during evolving omicron surgesJohn Schrom, Carina Marquez, Chung-Yu Wang, Aditi Saxena, Anthea M Mitchell, Salu Ribeiro, Genay Pilarowski, Robert Nakamura, Susana Rojas, Douglas Black, Maria G Contreras Oseguera, Edgar Castellanos Diaz, Joselin Payan, Susy Rojas, Diane Jones, Valerie Tulier-Laiwa, Aleks Zavaleta, Jacqueline Martinez, Gabriel Chamie, Carol Glaser, Kathy Jacobson, Maya Petersen, Joseph Derisi, and Diane V HavlirPloS one, 2023
Field assessment of BinaxNOW antigen tests as COVID-19 treatment entry point at a community testing site in San Francisco during evolving omicron surgesJohn Schrom, Carina Marquez, Chung-Yu Wang, Aditi Saxena, Anthea M Mitchell, Salu Ribeiro, Genay Pilarowski, Robert Nakamura, Susana Rojas, Douglas Black, Maria G Contreras Oseguera, Edgar Castellanos Diaz, Joselin Payan, Susy Rojas, Diane Jones, Valerie Tulier-Laiwa, Aleks Zavaleta, Jacqueline Martinez, Gabriel Chamie, Carol Glaser, Kathy Jacobson, Maya Petersen, Joseph Derisi, and Diane V HavlirPloS one, 2023COVID-19 oral treatments require initiation within 5 days of symptom onset. Although antigen tests are less sensitive than RT-PCR, rapid results could facilitate entry to treatment. We col- lected anterior nasal swabs for BinaxNOW and RT-PCR testing and clinical data at a walk- up, community site in San Francisco, California between January and June 2022. SARS- CoV-2 genomic sequences were generated from positive samples and classified according to subtype and variant. Monte Carlo simulations were conducted to estimate the expected proportion of SARS-CoV-2 infected persons who would have been diagnosed within 5 days of symptom onset using RT-PCR versus BinaxNOW testing. Among 25,309 persons tested with BinaxNOW, 2,799 had concomitant RT-PCR. 1137/2799 (40.6%) were SARS-CoV-2 RT-PCR positive. We identified waves of predominant omicron BA.1, BA.2, BA.2.12, BA.4, and BA.5 among 720 sequenced samples. Among 1,137 RT-PCR positive samples, 788/ 1137 (69%) were detected by BinaxNOW; 94% (669/711) of those with Ct value <30 were detected by BinaxNOW. BinaxNOW detection was consistent over lineages. In analyses to evaluate entry to treatment, BinaxNOW detected 81.7% (361/442, 95% CI: 77–85%) of per- sons with COVID-19 within 5 days of symptom onset. In comparison, RT-PCR (24-hour turn- around) detected 84.2% (372/442, 95% CI: 80–87%) and RT-PCR (48-hour turnaround) detected 67.0% (296/442, 95% CI: 62–71%) of persons with COVID-19 within 5 days of symptom onset. BinaxNOW detected high viral load from anterior nasal swabs consistently across omicron sublineages emerging between January and June of 2022. Simulations sup- port BinaxNOW as an entry point for COVID-19 treatment in a community field setting.
2022
- article
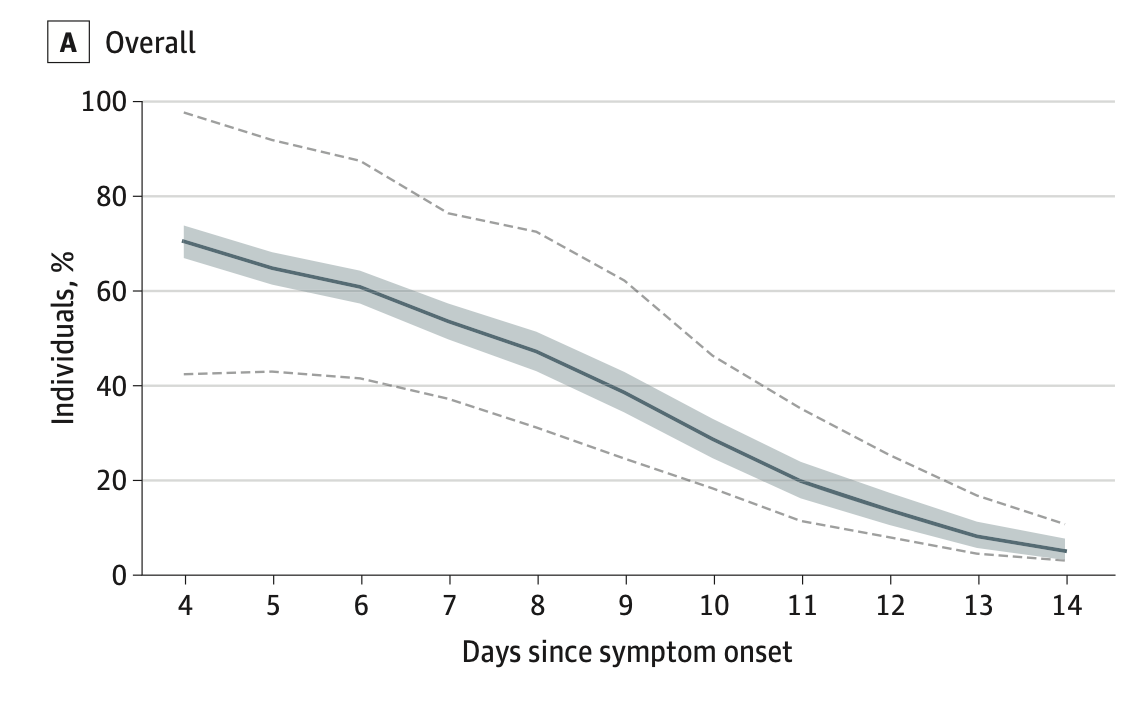 COVID-19 symptoms and duration of direct antigen test positivity at a community testing and surveillance site, January 2021-2022Carina Marquez, Andrew D Kerkhoff, John Schrom, Susana Rojas, Douglas Black, Anthea Mitchell, Chung-Yu Wang, Genay Pilarowski, Salustiano Ribeiro, Diane Jones, Joselin Payan, Simone Manganelli, Susy Rojas, Jonathan Lemus, Vivek Jain, Gabriel Chamie, Valerie Tulier-Laiwa, Maya Petersen, Joseph DeRisi, and Diane HavlirJAMA Network Open, 2022
COVID-19 symptoms and duration of direct antigen test positivity at a community testing and surveillance site, January 2021-2022Carina Marquez, Andrew D Kerkhoff, John Schrom, Susana Rojas, Douglas Black, Anthea Mitchell, Chung-Yu Wang, Genay Pilarowski, Salustiano Ribeiro, Diane Jones, Joselin Payan, Simone Manganelli, Susy Rojas, Jonathan Lemus, Vivek Jain, Gabriel Chamie, Valerie Tulier-Laiwa, Maya Petersen, Joseph DeRisi, and Diane HavlirJAMA Network Open, 2022Importance: Characterizing the clinical symptoms and evolution of community-based SARS-CoV-2 infections may inform health practitioners and public health officials in a rapidly changing landscape of population immunity and viral variants. Objectives: To compare COVID-19 symptoms among people testing positive with a rapid antigen test (RAT) during the Omicron BA.1 variant period (December 1, 2021, to January 30, 2022) with the pre-Delta (January 10 to May 31, 2021) and Delta (June 1 to November 30, 2021) variant periods and to assess the duration of RAT positivity during the Omicron BA.1 surge. Design, Setting, and Participants: This cross-sectional study was conducted from January 10, 2021, to January 31, 2022, at a walk-up community COVID-19 testing site in San Francisco, California. Participants included children and adults seeking COVID-19 testing with an RAT, regardless of age, vaccine status, or symptoms. Main Outcomes and Measures: Fisher exact tests or χ2 tests were used to compare COVID-19 symptoms during the Omicron BA.1 period with the pre-Delta and Delta periods for vaccination status and age group. Among people returning for repeated testing during the Omicron period, the proportion with a positive RAT between 4 and 14 days from symptom onset or since first positive test if asymptomatic was estimated. Results: Among 63,277 persons tested (median [IQR] age, 32 [21-44] years, with 12.0% younger than 12 years; 52.0% women; and 68.5% Latinx), a total of 18 301 people (28.9%) reported symptoms, of whom 4565 (24.9%) tested positive for COVID-19. During the Omicron BA.1 period, 3032 of 7283 symptomatic participants (41.6%) tested positive, and the numbers of these reporting cough and sore throat were higher than during pre-Delta and Delta periods (cough: 2044 [67.4%] vs 546 [51.3%] of 1065 participants, P < .001 for pre-Delta, and 281 [60.0%] of 468 participants, P = .002, for Delta; sore throat: 1316 [43.4%] vs 315 [29.6%] of 1065 participants, P < .001 for pre-Delta, and 136 [29.1%] of 468 participants, P < .001, for Delta). Compared with the 1065 patients with positive test results in the pre-Delta period, congestion among the 3032 with positive results during the Omicron BA.1 period was more common (1177 [38.8%] vs 294 [27.6%] participants, P < .001), and loss of taste or smell (160 [5.3%] vs 183 [17.2%] participants, P < .001) and fever (921 [30.4%] vs 369 [34.7%] participants, P = .01) were less common. In addition, during the Omicron BA.1 period, fever was less common among the people with positive test results who had received a vaccine booster compared with those with positive test results who were unvaccinated (97 [22.5%] of 432 vs 42 [36.2%] of 116 participants, P = .003), and fever and myalgia were less common among participants who had received a booster compared with those with positive results who had received only a primary series (fever: 97 [22.5%] of 432 vs 559 [32.8%] of 1705 participants, P < .001; myalgia: 115 [26.6%] of 432 vs 580 [34.0%] of 1705 participants, P = .003). During the Omicron BA.1 period, 5 days after symptom onset, 507 of 1613 people (31.1%) with COVID-19 stated that their symptoms were similar, and 95 people (5.9%) reported worsening symptoms. Among people testing positive, 80.2% of participants who were symptomatic and retested remained positive 5 days after symptom onset. Conclusions and Relevance: In this cross-sectional study, COVID-19 upper respiratory tract symptoms were more commonly reported during the Omicron BA.1 period than during the pre-Delta and Delta periods, with differences by vaccination status. Rapid antigen test positivity remained high 5 days after symptom onset, supporting guidelines requiring a negative test to inform the length of the isolation period.
- article
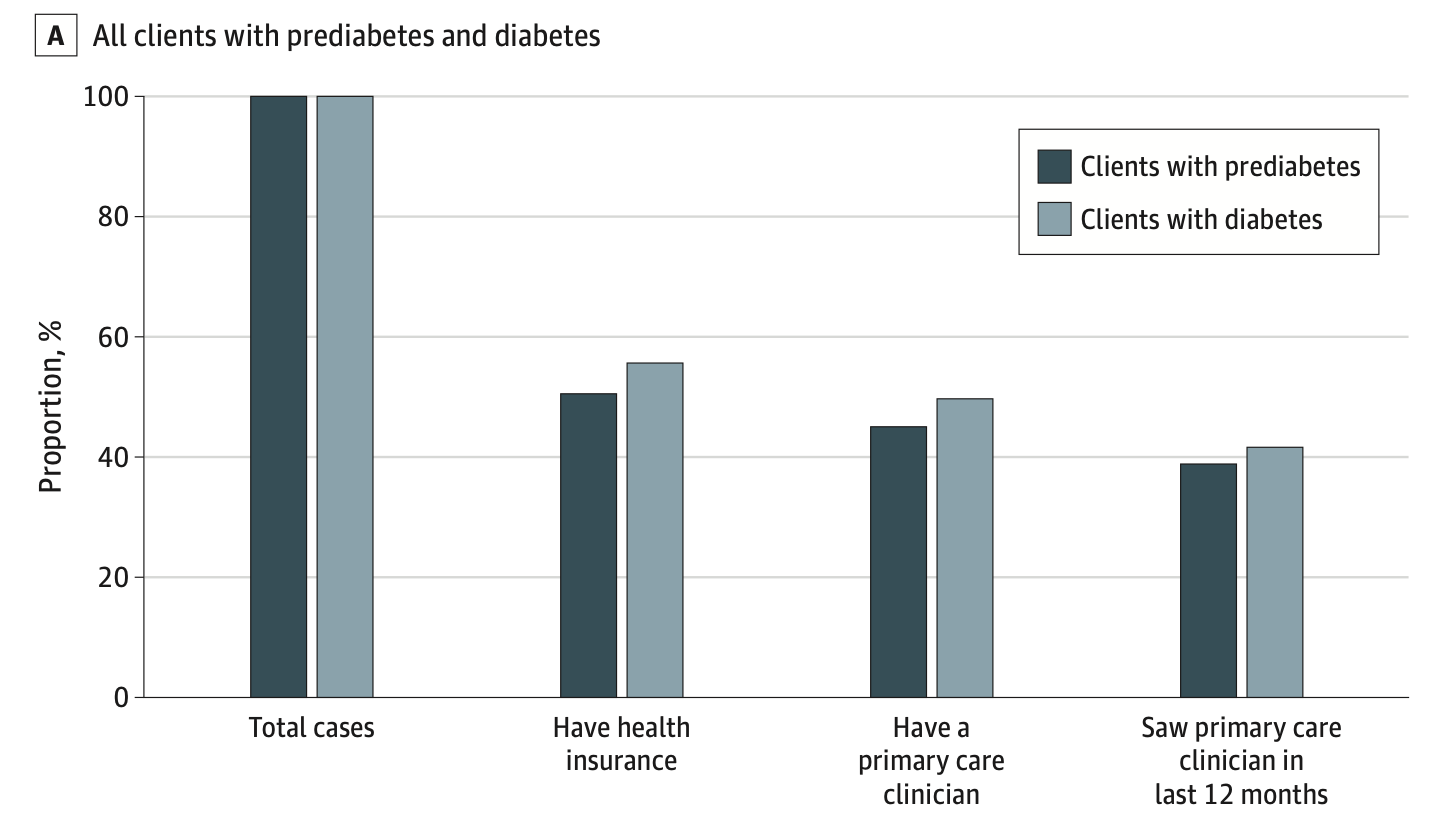 Integrating rapid diabetes screening into a latinx focused community-based low-barrier COVID-19 testing programAndrew D Kerkhoff, Susana Rojas, Douglas Black, Salustiano Ribeiro, Susy Rojas, Rebecca Valencia, Jonathan Lemus, Joselin Payan, John Schrom, Diane Jones, Simone Manganelli, Shalom Bandi, Gabriel Chamie, Valerie Tulier-Laiwa, Maya Petersen, Diane Havlir, and Carina MarquezJAMA Network Open, 2022
Integrating rapid diabetes screening into a latinx focused community-based low-barrier COVID-19 testing programAndrew D Kerkhoff, Susana Rojas, Douglas Black, Salustiano Ribeiro, Susy Rojas, Rebecca Valencia, Jonathan Lemus, Joselin Payan, John Schrom, Diane Jones, Simone Manganelli, Shalom Bandi, Gabriel Chamie, Valerie Tulier-Laiwa, Maya Petersen, Diane Havlir, and Carina MarquezJAMA Network Open, 2022Importance: Community-based COVID-19 testing and vaccination programs play a crucial role in mitigating racial and ethnic disparities in COVID-19 service delivery. They also represent a platform that can be leveraged to expand access to testing for chronic diseases, including diabetes, that disproportionately affect the Latinx community and other marginalized communities. Objective: To evaluate outcomes associated with a diabetes testing strategy designed to reach low-income Latinx persons by leveraging COVID-19 testing infrastructure and community trust developed during the COVID-19 pandemic. Design, Setting, and Participants: This health care improvement study was conducted from August 1 to October 5, 2021, at an outdoor, community-based COVID-19 testing site at a transport hub in the Mission Neighborhood in San Francisco, California. Because the program was designed to expand access to diabetes screening to the local community, all individuals presenting for on-site testing were eligible. Data were analyzed in November 2021. Interventions: Integration of rapid, point-of-care hemoglobin A1c screening as a testing option in an existing low-barrier COVID-19 testing program. Main Outcomes and Measures: Evaluation was guided by the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) framework and utilized programmatic data and structured surveys among clients and staff. Results: Of 6631 individuals tested (median [IQR] age 39.3 [29.7-51.3] years; 3417 [52.3%] female, 4348 [65.6%] Latinx), 923 (13.9%) underwent hemoglobin A1c testing with or without COVID-19 testing and 5708 (86.1%) underwent COVID-19 testing only. Individuals tested for diabetes were more likely to be Latinx (763 of 923 individuals [82.7%] who underwent testing were Latinx vs 3585 of 5708 [62.8%] not undergoing testing), have an annual household income of less than $50 000 (450 individuals [81.2%] vs 2409 individuals [66.0%]), and not have health insurance (381 individuals [47.2%] vs 1858 individuals [39.9%]), and 206 (48.0%) had never tested for diabetes before. Overall, 313 (33.9%) and 113 (12.2%) individuals had prediabetes and diabetes, respectively; only 141 of 354 of these individuals (39.8%) had a primary care clinician whom they had seen in the prior 12 months, which was lower among Latinx individuals (113 of 307 individuals [36.8%] vs 28 of 47 [59.6%]). Acceptability of the rapid testing program was high—98% were satisfied with their visit and 96% said they would return for future services; key factors underpinning acceptability included friendly staff, efficiency, and a convenient location. Conclusions and Relevance: In this health care improvement study conducted within an existing community-based COVID-19 testing program, integrating rapid testing for diabetes was feasible, reached low-income Latinx individuals, and identified many persons with prediabetes and diabetes, most of whom lacked access to services in formal health care settings. Leveraging pandemic-related public health responses represents an important opportunity for engaging socioeconomically disadvantaged populations into care for diabetes.
- article
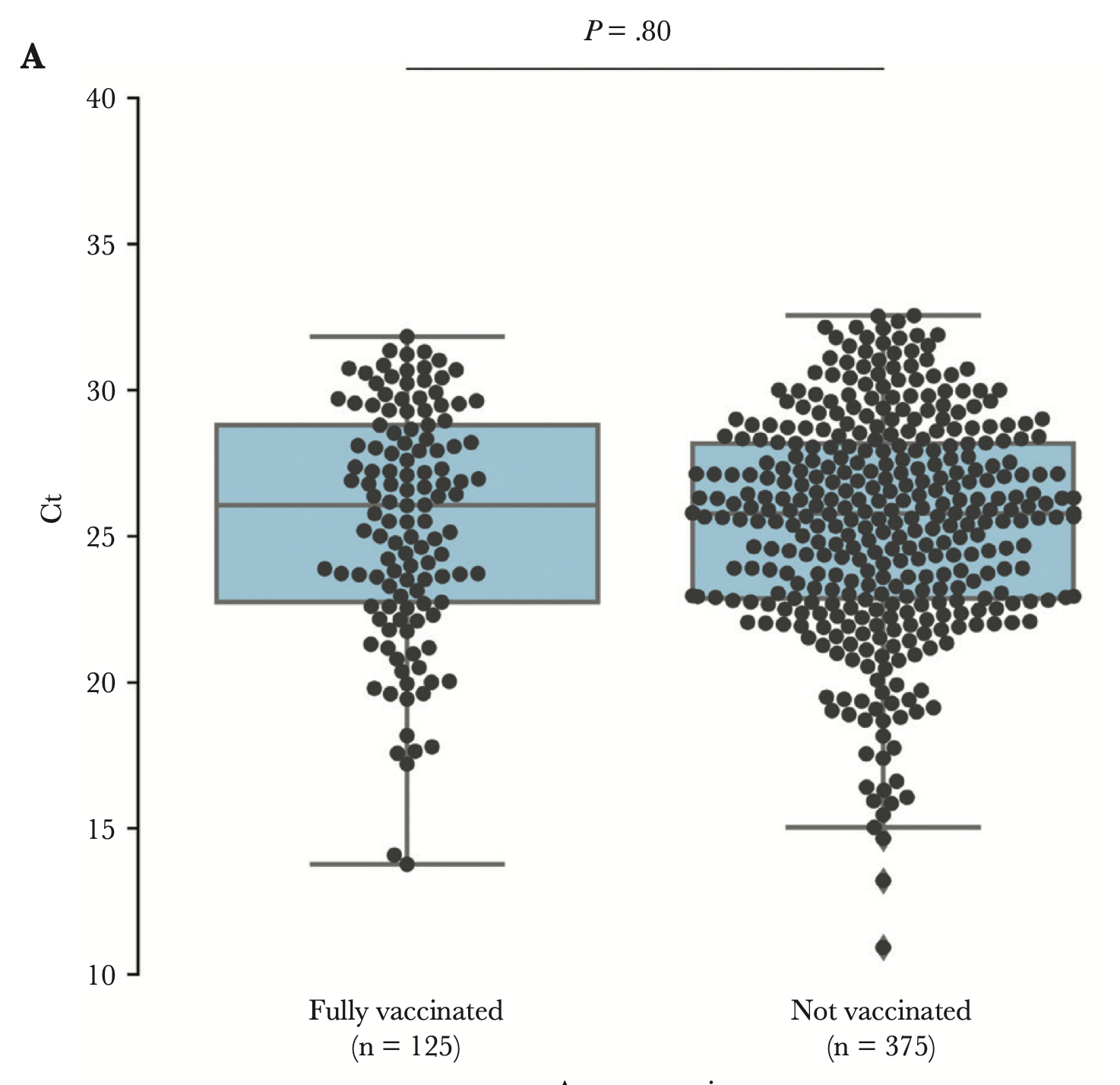 Viral load among vaccinated and unvaccinated, asymptomatic and symptomatic persons infected with the SARS-CoV-2 delta variantCharlotte B Acharya, John Schrom, Anthea M Mitchell, David A Coil, Carina Marquez, Susana Rojas, Chung Yu Wang, Jamin Liu, Genay Pilarowski, Leslie Solis, Elizabeth Georgian, Sheri Belafsky, Maya Petersen, Joesph DeRisi, Richard Michelmore, and Diane HavlirOpen Forum Infectious Diseases, 2022
Viral load among vaccinated and unvaccinated, asymptomatic and symptomatic persons infected with the SARS-CoV-2 delta variantCharlotte B Acharya, John Schrom, Anthea M Mitchell, David A Coil, Carina Marquez, Susana Rojas, Chung Yu Wang, Jamin Liu, Genay Pilarowski, Leslie Solis, Elizabeth Georgian, Sheri Belafsky, Maya Petersen, Joesph DeRisi, Richard Michelmore, and Diane HavlirOpen Forum Infectious Diseases, 2022We found no significant difference in cycle threshold values between vaccinated and unvaccinated persons infected with severe acute respiratory syndrome coronavirus 2 Delta, overall or stratified by symptoms. Given the substantial proportion of asymptomatic vaccine breakthrough cases with high viral levels, interventions, including masking and testing, should be considered in settings with elevated coronavirus disease 2019 transmission.
- article
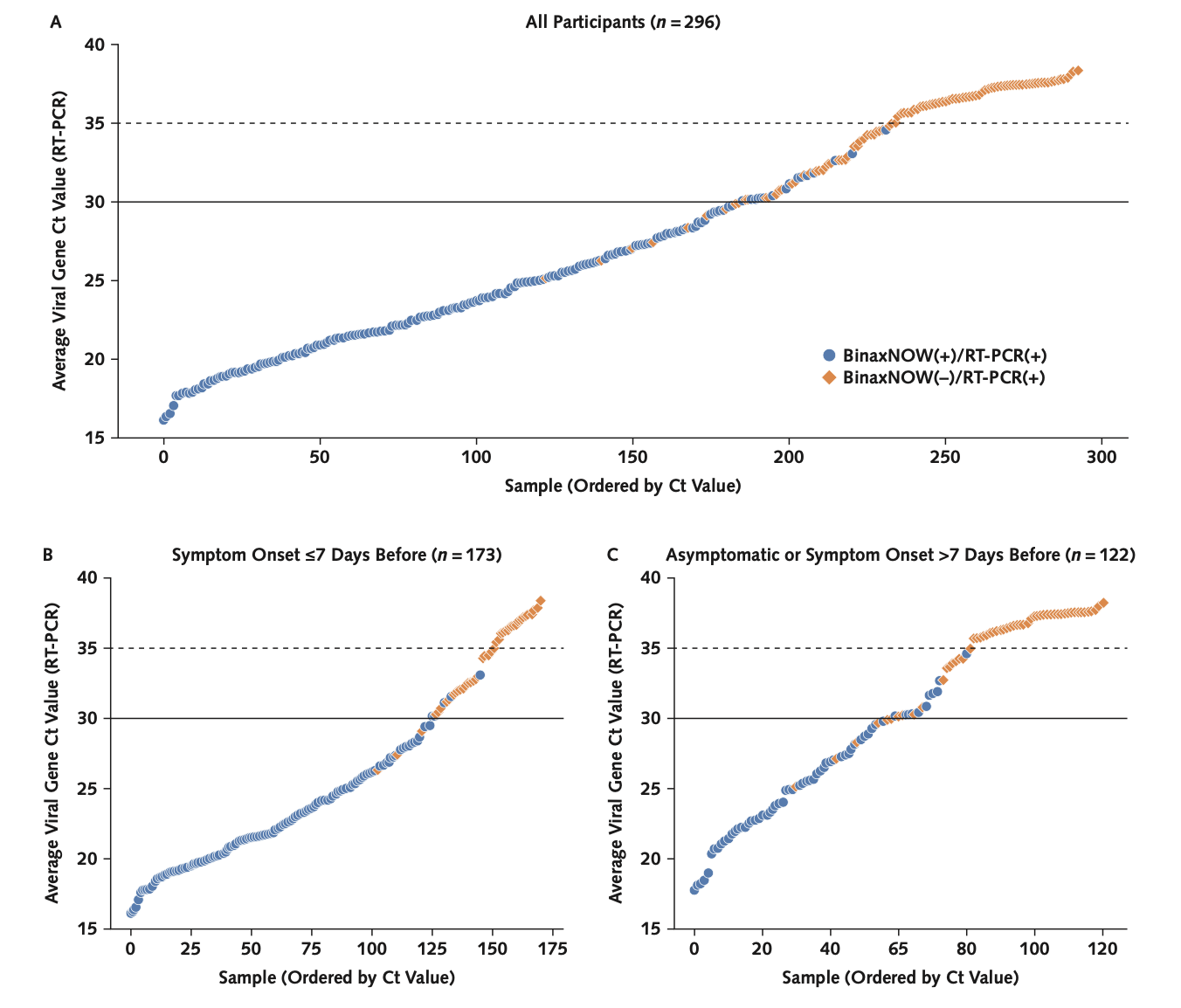 Comparison of SARS-CoV-2 reverse transcriptase polymerase chain reaction and BinaxNOW rapid antigen tests at a community site during an Omicron surge: a cross-sectional studyJohn Schrom, Carina Marquez, Genay Pilarowski, Chung-Yu Wang, Anthea Mitchell, Robert Puccinelli, Doug Black, Susana Rojas, Salustiano Ribeiro, Valerie Tulier-Laiwa, Jacqueline Martinez, Joselin Payan, Susy Rojas, Diane Jones, Daniel Martinez, Robert Nakamura, Gabriel Chamie, Vivek Jain, Maya Petersen, Joseph Derisi, and Diane HavlirAnnals of internal medicine, 2022
Comparison of SARS-CoV-2 reverse transcriptase polymerase chain reaction and BinaxNOW rapid antigen tests at a community site during an Omicron surge: a cross-sectional studyJohn Schrom, Carina Marquez, Genay Pilarowski, Chung-Yu Wang, Anthea Mitchell, Robert Puccinelli, Doug Black, Susana Rojas, Salustiano Ribeiro, Valerie Tulier-Laiwa, Jacqueline Martinez, Joselin Payan, Susy Rojas, Diane Jones, Daniel Martinez, Robert Nakamura, Gabriel Chamie, Vivek Jain, Maya Petersen, Joseph Derisi, and Diane HavlirAnnals of internal medicine, 2022Background: SARS-CoV-2 rapid antigen tests are an important public health tool Objective: To evaluate field performance of the BinaxNOW rapid antigen test (Abbott) compared with reverse transcriptase polymerase chain reaction (RT-PCR) for detecting infection with the Omicron variant of SARS-CoV-2. Design: Cross-sectional surveillance study. Setting: Free, walk-up, outdoor, urban community testing and vaccine site led by Unidos en Salud, serving a predominantly Latinx community highly impacted by COVID-19. Participants: Persons seeking COVID-19 testing in January 2022. Measurements: Simultaneous BinaxNOW and RT-PCR from nasal, cheek, and throat swabs, including cycle threshold (Ct) measures; a lower Ct value is a surrogate for higher amounts of virus. Results: Among 731 persons tested with nasal swabs, there were 296 (40.5%) positive results on RT-PCR; 98.9% were the Omicron variant. BinaxNOW detected 95.2% (95% CI, 91% to 98%) of persons who tested positive on RT-PCR with a Ct value below 30, 82.1% (CI, 77% to 87%) of those who tested positive on RT-PCR with a Ct value below 35, and 65.2% (CI, 60% to 71%) of all who were positive on RT-PCR. Among 75 persons with simultaneous nasal and cheek swabs, BinaxNOW using a cheek swab failed to detect 91% (20 of 22) of specimens that were positive on BinaxNOW with a nasal swab. Among persons with simultaneous nasal and throat swabs who were positive on RT-PCR with a Ct value below 30, 42 of 49 (85.7%) were detected by nasal BinaxNOW, 23 of 49 (46.9%) by throat BinaxNOW, and 44 of 49 (89.8%) by either. Limitation: Participants were a cross-sectional sample from a community-based sentinel surveillance site, precluding study of viral or symptom dynamics. Conclusion: BinaxNOW detected persons with high SARS-CoV-2 levels during the Omicron surge, enabling rapid responses to positive test results. Cheek or throat swabs should not replace nasal swabs. As currently recommended, high-risk persons with an initial negative BinaxNOW result should have repeated testing.
2020
- poster
 Predicting Discrete Reason for Visit using Personalized PageRankJohn Schrom*, Emma Sahn, Bobby Caplin, and Vijan JoshiIn AMIA, 2020
Predicting Discrete Reason for Visit using Personalized PageRankJohn Schrom*, Emma Sahn, Bobby Caplin, and Vijan JoshiIn AMIA, 2020Patients generally provide a reason for visit when booking a primary care appointment. This reason is useful for provisioning care, monitoring utilization, forecasting future patient and clinic needs, and assessing risk. There are a finite set of reasons patients seek primary care, and many are often readily apparent (e.g., follow-up from a recent hospitalization). Tethered personal health records increasingly allow patients to book their own appointments online, creating opportunities for improved user experience and enhanced data collection. This preliminary work seeks to understand the feasibility of predicting a patient’s reason for visit prior to appointment booking, in order to help improve the user experience of booking appointments via a personal health record.
- oral
 The Impact of Data Communication Style in Email-Based Quality Reports on Depression Screening in Primary CareJohn Schrom*, Ariel Slam, Jess Liu, Colleen Bouey, Laura Berk, Alli Gilmore, Lenny Lesser, and Raj BehalIn AMIA Summit, 2020
The Impact of Data Communication Style in Email-Based Quality Reports on Depression Screening in Primary CareJohn Schrom*, Ariel Slam, Jess Liu, Colleen Bouey, Laura Berk, Alli Gilmore, Lenny Lesser, and Raj BehalIn AMIA Summit, 2020Over 16 million adults suffer from a major depressive episode each year[1], and depression remains a leading cause of disability among people over 15 years of age. An estimated two-thirds of patients with depression are not diagnosed[2]. To address this issue, our national primary care clinic system launched routine depression screening at wellness visits via a quality improvement collaborative model. In launching this collaborative, we also sought to understand the most impactful method of communicating quality measure data to clinic staff. Two hypotheses emerged during this program design around how to increase the depression screening rate: provide specific and directive information to clinic staff regarding what action is necessary to improve their quality measure rates (e.g., “Screen 70 patients for depression”), versus providing the quality measure rate and associated trend information and allowing the staff to interpret and act accordingly (e.g., “Your depression screening rate is 20%”).
2019
- poster
 Modifying the Order of Medication Search Results in an Electronic Health Record to Increase Physician Generic Prescribing BehaviorJohn Schrom*, Paul Cohen, Sophie Krisch, and Tamer FakhouriIn AMIA Summit, 2019
Modifying the Order of Medication Search Results in an Electronic Health Record to Increase Physician Generic Prescribing BehaviorJohn Schrom*, Paul Cohen, Sophie Krisch, and Tamer FakhouriIn AMIA Summit, 2019Prescription drug costs accounted for 10% of national health expenditure in the United States in 2016. Generic drugs have the potential to eliminate significant spending when appropriately substituted, and are associated with greater patient adherence. Malhotra et. al. showed that reengineering an e-prescribing interface to convert providers’ selections of branded drugs to generic drugs significantly increased the proportion of generic prescriptions. However, this approach may not be appropriate for other workflows, such as when providers must choose between non-equivalent options. We present a simple intervention whereby generic options are promoted within search results while still allowing providers to choose brand or generic options. The intervention significantly increased the rate of generic prescribing without requiring either changes to the design of the ordering module or dedicated provider communication.
- poster
 Associating Chief Complaints with Electronic Health Record Activity to Decrease Provider Administrative BurdenJohn Schrom*, Vijan Joshi, Colleen Bouey, and Allison GilmoreIn AMIA, 2019
Associating Chief Complaints with Electronic Health Record Activity to Decrease Provider Administrative BurdenJohn Schrom*, Vijan Joshi, Colleen Bouey, and Allison GilmoreIn AMIA, 2019Physician burnout has become a widespread issue, with electronic health record (EHR) use known to be a major predictor of burnout[1]. The burden of EHRs has become so problematic that patients are now reluctant to engage in medical encounters due to the overuse of technology in the exam room[2]. The average physician spends almost six hours per day interacting with their EHR[3]. Machine learning and data mining can decrease this administrative burden through smart shortcuts, based on predicted user behavior.
- oral
 Design and Implementation of an Electronic Survey for Follow-Up of Acute Conditions in Primary CareVijan Joshi*, John Schrom, Kyle Munkittrick, Carlo Stearns, Svetlana Ivanova, David Hoang, Lenard Lesser, Tamer Fakhouri, and Andrew DiamondIn AMIA, 2019
Design and Implementation of an Electronic Survey for Follow-Up of Acute Conditions in Primary CareVijan Joshi*, John Schrom, Kyle Munkittrick, Carlo Stearns, Svetlana Ivanova, David Hoang, Lenard Lesser, Tamer Fakhouri, and Andrew DiamondIn AMIA, 2019Acute conditions make up a large portion of the reason for visits to primary care practices. These acute conditions can sometimes progress to more serious illnesses. Resource limitations make scheduling follow-up visits, calls or messages to check for resolution of these conditions impractical. Awareness of patient outcomes helps prevent diagnostic delays, and engaging patients to seek follow-up care if they are not improving creates a diagnostic safety net. This initial study aimed to assess provider and patient engagement in an electronic follow up survey after a primary care encounter for an acute condition.
2018
- poster
 Identifying Non-Clinical Patient Messages Using Naive BayesPhilip Ingram, Rom Srinivasan, Patrick Grennan, and John Schrom*In AMIA, 2018
Identifying Non-Clinical Patient Messages Using Naive BayesPhilip Ingram, Rom Srinivasan, Patrick Grennan, and John Schrom*In AMIA, 2018Physician time and attention is an increasingly scarce resource. Primary care physicians spend nearly two hours in the electronic health record for every hour of patient care, with inbox management alone accounting for 85 minutes of their work day[1]. Further, physician availability via email has been shown to increase patient email volume by over 300%[2]. This creates a substantial burden on physicians, forcing them to choose between being accessible to patients, completing their administrative responsibilities, and maintaining their own time for personal wellbeing. This preliminary study attempts to assess the feasibility of identifying and rerouting non-clinical messages to administrative staff (“admins”) in order to decrease the burden on physicians.
- poster
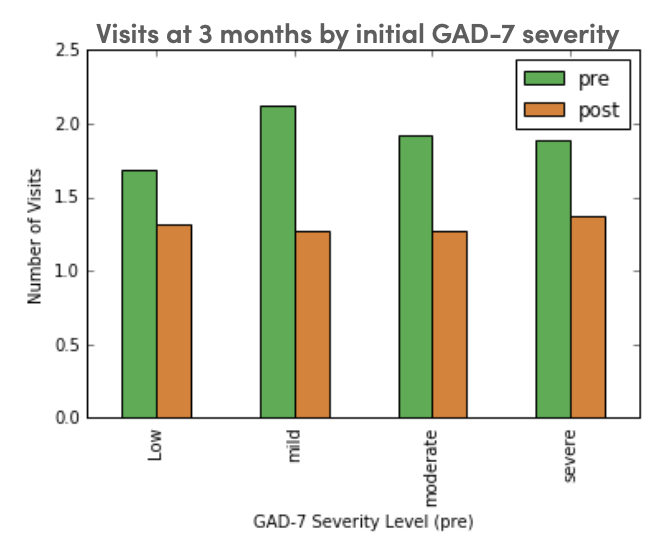 Group Visits Improve Symptoms and Lower Utilization in Primary Care Patients with AnxietyJohn Schrom*, Kareen Patterson, Allison Gilmore, Paul Cohen, and Lenard LesserIn Academy Health, 2018
Group Visits Improve Symptoms and Lower Utilization in Primary Care Patients with AnxietyJohn Schrom*, Kareen Patterson, Allison Gilmore, Paul Cohen, and Lenard LesserIn Academy Health, 2018We developed a four-week, in-person, group visit program which emphasizes a skills- based approach to stress and anxiety management with integrated health coaching for sustainable behavior change. The program is co-led by a clinician and health coach, and teaches techniques informed by yoga, mindfulness meditation, and cognitive behavioral therapy. We administered symptom surveys, including the Generalized Anxiety Disorder-7 item scale (GAD-7), before and after the program; care utilization was monitored via our electronic health record. We evaluated change in anxiety symptoms and health-care utilization at three and six months, using a pre-/post- study design with paired t-tests. All participants who attended at least one session were included; only those with sufficient utilization data were included in the care utilization analysis.
2013
- oral
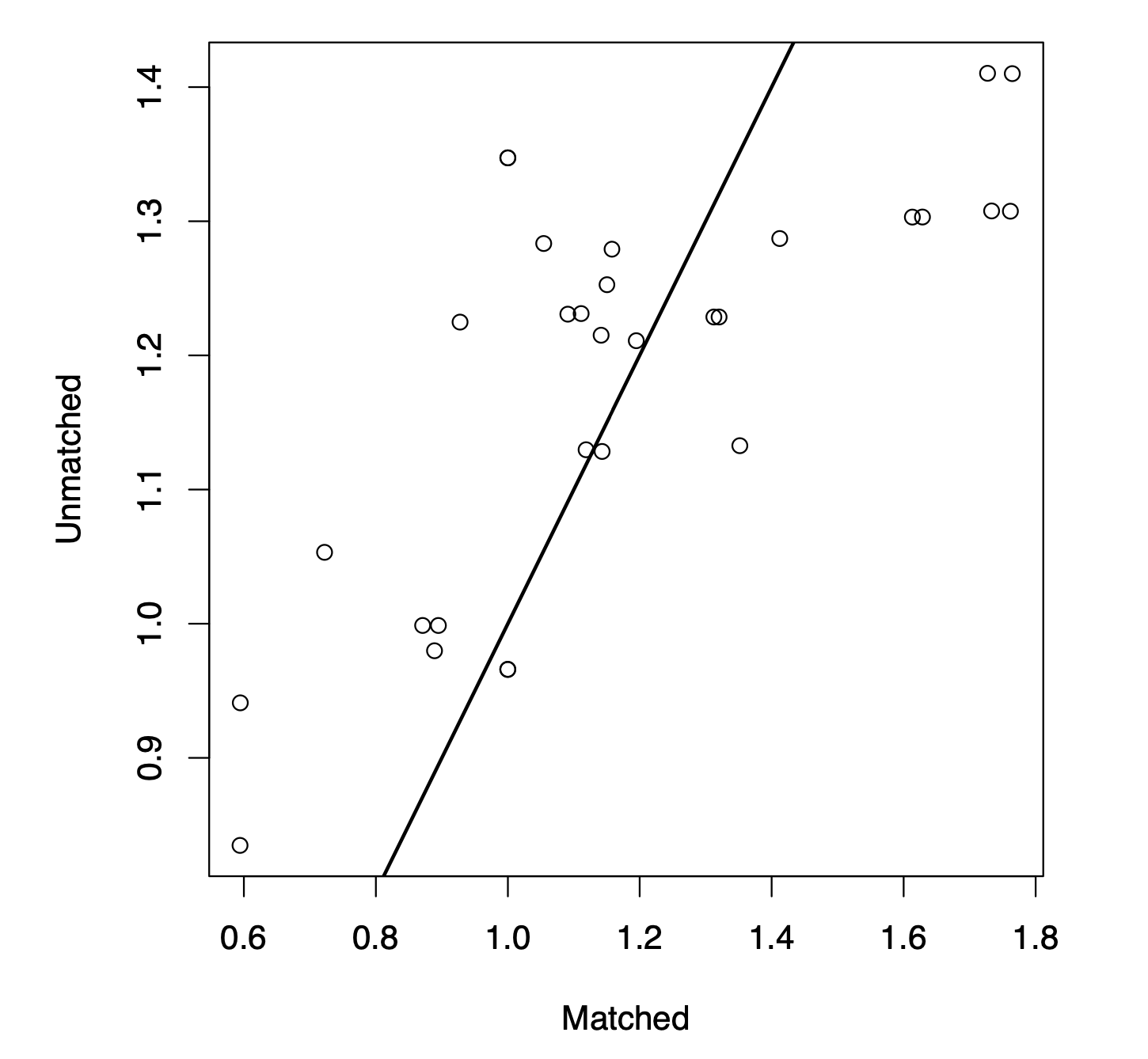 Quantifying the effect of statin use in pre-diabetic phenotypes discovered through association rule miningJohn Schrom*, Pedro J Caraballo, M Regina Castro, and Gyorgy J SimonIn AMIA Annual Symposium Proceedings, 2013
Quantifying the effect of statin use in pre-diabetic phenotypes discovered through association rule miningJohn Schrom*, Pedro J Caraballo, M Regina Castro, and Gyorgy J SimonIn AMIA Annual Symposium Proceedings, 2013Prediabetes is the most important risk factor for developing type-2 diabetes mellitus, an important and growing epidemic. Prediabetes is often associated with comorbidities including hypercholesterolemia. While statin drugs are indicated to treat hypercholesterolemia, recent reports suggest a possible increased risk of developing overt diabetes associated with the use of statins. Association rule mining is a data mining technique capable of identifying interesting relationships between risks and treatments. However, it is limited in its ability to accurately calculate the effect of a treatment, as it does not appropriately account for bias and confounding. We propose a novel combination of propensity score matching and association rule mining to account for this bias, and find meaningful associations between a treatment and outcome for various subpopulations. We demonstrate this technique on a real diabetes data set examining the relationship between statin use and diabetes, and identify risk and protective factors previously not clearly defined.
- oral
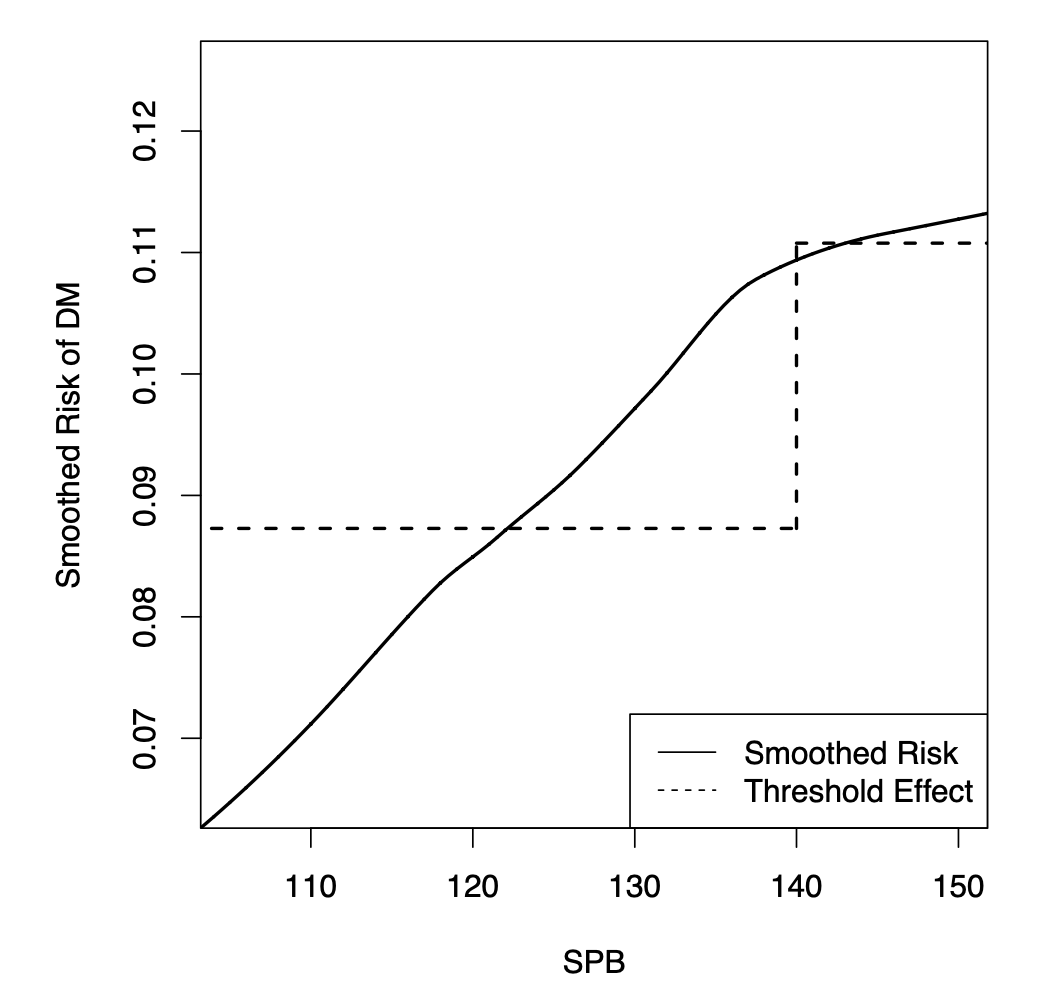 Survival association rule mining towards type 2 diabetes risk assessmentGyorgy J Simon*, John Schrom, M Regina Castro, Peter W Li, and Pedro J CaraballoIn AMIA annual symposium proceedings, 2013
Survival association rule mining towards type 2 diabetes risk assessmentGyorgy J Simon*, John Schrom, M Regina Castro, Peter W Li, and Pedro J CaraballoIn AMIA annual symposium proceedings, 2013Type-2 Diabetes Mellitus is a growing epidemic that often leads to severe complications. Effective preventive measures exist and identifying patients at high risk of diabetes is a major health-care need. The use of association rule mining (ARM) is advantageous, as it was specifically developed to identify associations between risk factors in an interpretable form. Unfortunately, traditional ARM is not directly applicable to survival outcomes and it lacks the ability to compensate for confounders and to incorporate dosage effects. In this work, we propose Survival Association Rule (SAR) Mining, which addresses these shortcomings. We demonstrate on a real diabetes data set that SARs are naturally more interpretable than the traditional association rules, and predictive models built on top of these rules are very competitive relative to state of the art survival models and substantially outperform the most widely used diabetes index, the Framingham score.
- oral
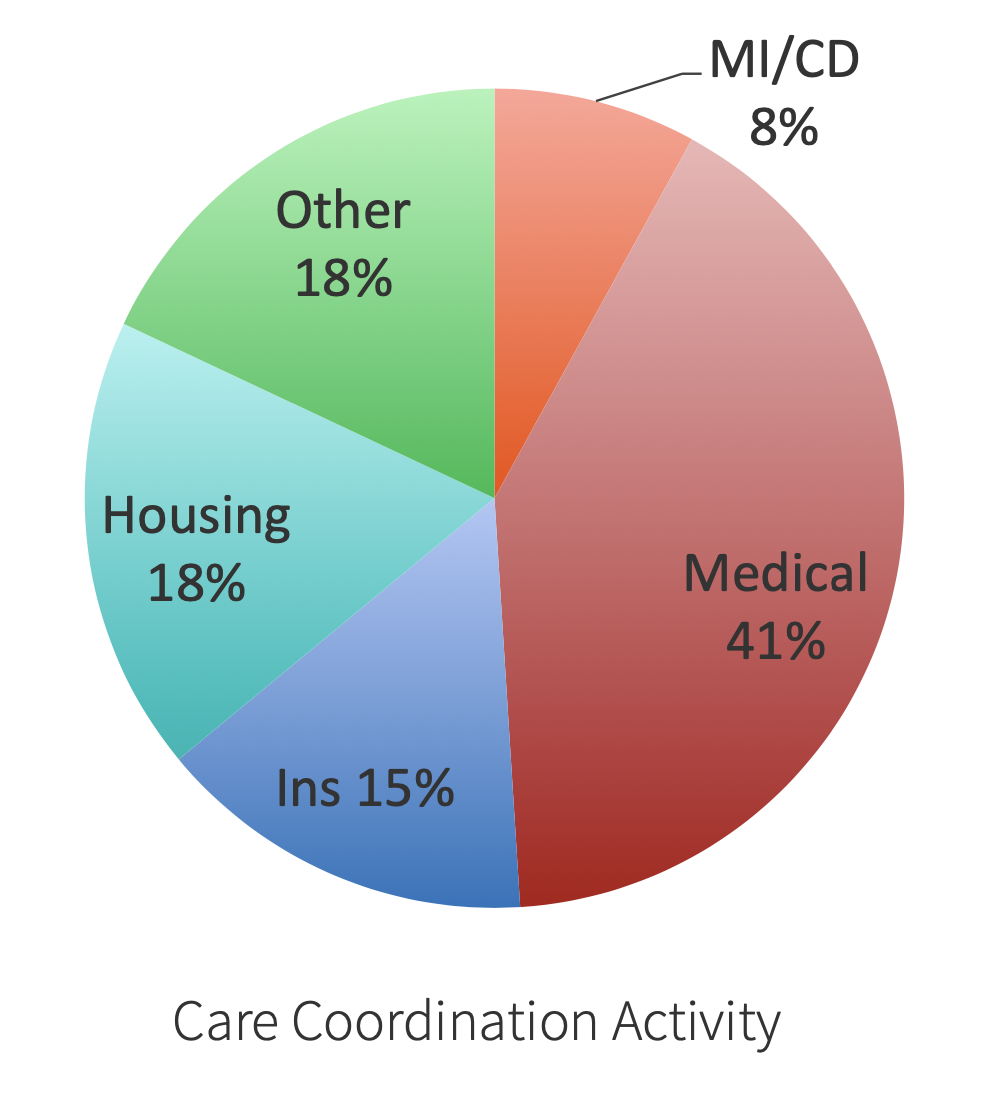 Predicting Care Coordination Utilization in Minnesota Health Care HomesJohn Schrom*, Scott Shimotsu, Rob Kreiger, Sara Poplau, Molly Jacques, and Kevin LarsenIn American Public Health Association, 2013
Predicting Care Coordination Utilization in Minnesota Health Care HomesJohn Schrom*, Scott Shimotsu, Rob Kreiger, Sara Poplau, Molly Jacques, and Kevin LarsenIn American Public Health Association, 2013In 2008, the Minnesota Legislature passed health care reform legislation that created the Health Care Homes model. This new care and payment approach encourages clinics to work with patients to provide better care coordination by providing a per-member, per-month payment based on a medical complexity tier measure for enrolled patients at certified clinics. To date, Hennepin County Medical Center (HCMC) has six certified Health Care Homes, serving over 750 patients. This study aims to validate the current medical complexity tier measure, as well as to identify how non-medical factors influence the utilization of care coordination services.
